We are a full-spectrum pharmaceutical consultancy providing strategic, scientific, and executional support across bioequivalence, clinical pharmacology, formulation development, and CRO selection. With over 17 years of hands-on industry experience and our own established CRO in India, we bridge the gap between regulatory intent and scientific execution—efficiently, compliantly, and cost-effectively.
Our clients range from emerging pharma startups to established global manufacturers, each supported with tailored, evidence-driven solutions.
Call to Action: Talk to a Pharma Consultant | Plan Your BA/BE Study | Find the Right CRO Partner




Integrated Pharma Consultancy Backed by 17+ Years of Scientific & Regulatory Excellence
A Consultancy Built by Scientists, Not Just Advisors
Our Core Consultancy Services








Bioavailability & Bioequivalence (BA/BE) Advisory
Clinical Pharmacology & Dosage Calculation Support
Formulation & Development Consultancy
CRO Identification, Qualification & Study Oversight
Bioavailability & Bioequivalence (BA/BE) Advisory
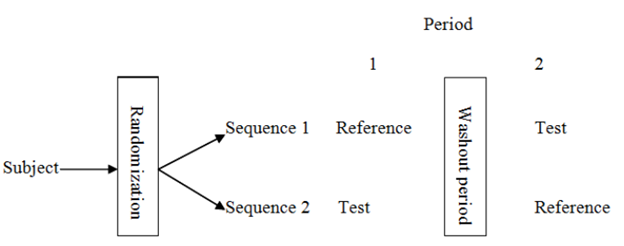
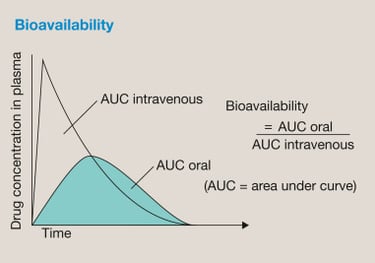



We support sponsors across the entire BA/BE lifecycle, from early planning to study completion:
BA/BE study strategy and protocol advisory
Selection of appropriate reference and test products
Sample size estimation and power calculations
Regulatory pathway guidance (India and global submissions)
CRO feasibility, budgeting, and timeline planning
Risk assessment and study optimization
Ideal for: Generic manufacturers, ANDA aspirants, emerging pharma companies.
Clinical Pharmacology & Dosage Calculation Support
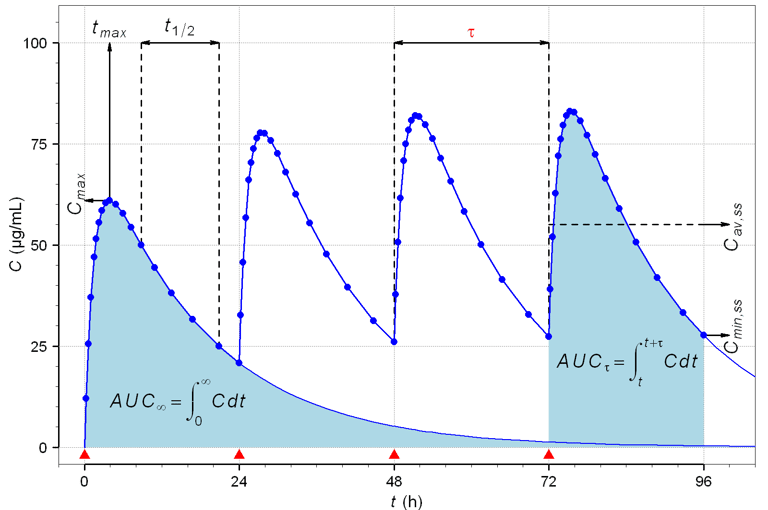
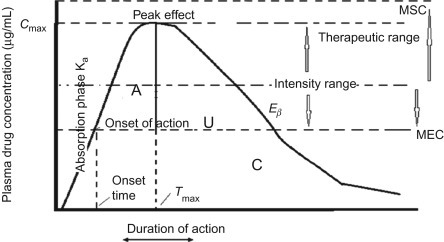
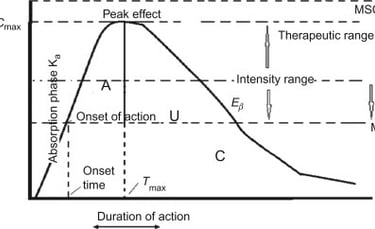
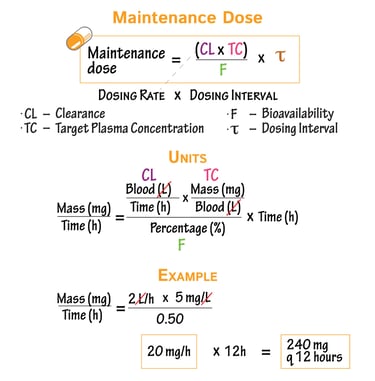
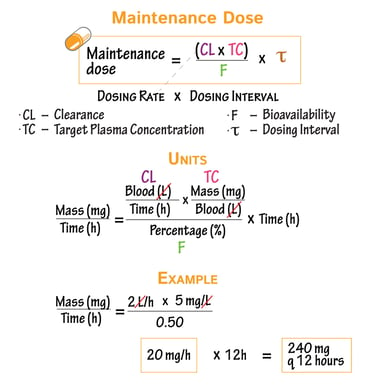
We provide scientifically rigorous and regulator-aligned guidance on:
Dose justification and dose selection
Pharmacokinetic and pharmacodynamic interpretations
Sampling schedule optimization
Food-effect and fasting study considerations
Bridging study requirements
This service is particularly valuable for complex generics, modified-release products, and narrow therapeutic index drugs.
Formulation & Development Consultancy




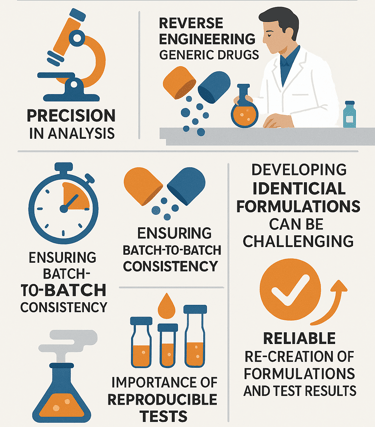
We assist formulation teams in addressing real-world development challenges, including:
Selection of appropriate excipients
Bio-relevant formulation strategy
Troubleshooting BE failures
Scale-up and comparability considerations
Modified-release and special dosage forms advisory
Our inputs are grounded in actual BA/BE outcomes, not just formulation theory.
CRO Identification, Qualification & Study Oversight
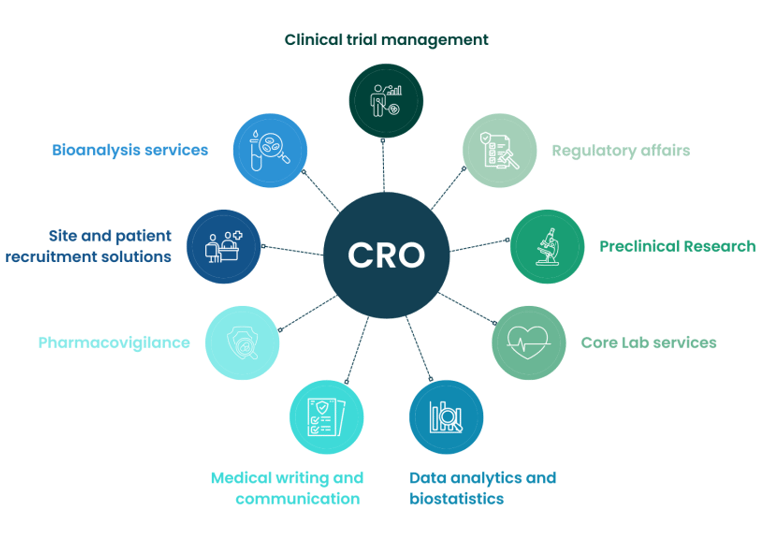
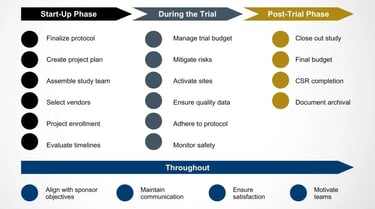
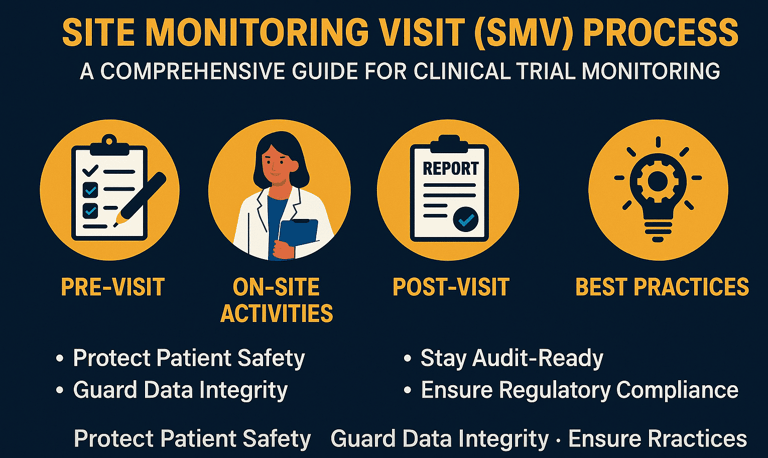

Choosing the right CRO is critical. We help sponsors:
Identify the most suitable CRO based on molecule, budget, and regulatory target
Conduct CRO qualification and technical due diligence
Review protocols, bioanalytical plans, and timelines
Provide independent study oversight and scientific governance
For clients who choose our in-house CRO in India, we offer seamless consultancy-to-execution transition, reducing handover risks and delays.
